Nicotinamide adenine dinucleotide (NAD) is an indispensable cofactor in cells. Therefore, NAD level variation leads to global yet difficult-to-predict biological responses. Recently, a group led by Prof. Zongbao K. ZHAO from Dalian Institute of Chemical Physics, Chinese Academy of Sciences, created the synthetase for non-natural coenzyme nicotinamide cytosine dinucleotide (NCD) and established NCD self-sufficient microbial phenotype. The work was just published on Nat. Commun.
To circumvent the limitation associated with NAD level fluctuation, the team led by Prof. Zhao has committed to developing non-natural cofactor-based systems. In previous studies, researchers synthesized NCD, successfully changed the cofactor preference of malic enzyme, lactate dehydrogenase, phosphite dehydrogenase and formate dehydrogenase to favor NCD over their natural cofactors (J. Am. Chem. Soc., 2011). By solving several X-ray crystal structures of NCD-favoring enzymes, the molecular bases of cofactor preference changes were unveiled (ACS Catal., 2019). Moreover, by overexpressing these enzymes and a dedicated nucleotide transporter in Escherichia coli, several NCD-mediated redox systems for selective energy transfer were established (ACS Catal., 2017; Angew. Chem. Int. Ed., 2020). However, a major challenging remains to take NCD from the environment by microbial cells.
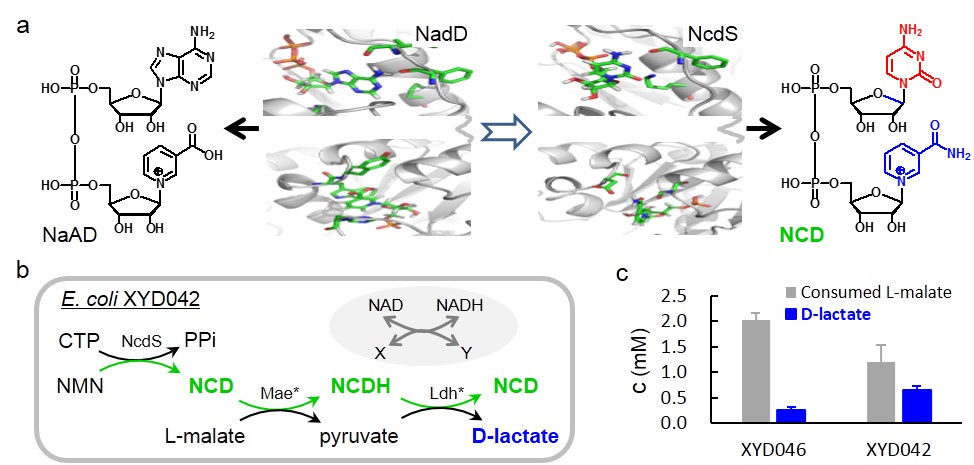
In this study, researchers created NCD synthetase (NcdS) by reprograming the ATP and nicotinic acid mononucleotide (NaMN) binding pockets of NaMN adenylyltransferase (NadD), the key enzyme in NAD biosynthesis, to favor CTP and nicotinamide mononucleotide (NMN). Upon overexpression of NcdS in E. coli, cells synthesized NCD and high cellular NCD level up to 5.0 mM was found when the flux of precursors was enhanced. Furthermore, by overexpression of NCD-linked malic enzyme and D-lactate dehydrogenase in those NCD self-sufficient hosts, highly selective conversion of L-malate to D-lactate was achieved. Therefore, NcdS together with NCD-linked enzymes may offer unique tools for intriguing studies in chemical biology and synthetic biology.
This work was supported by National Key R&D Program of China, National Natural Science Foundation of China and Dalian Institute of Chemical Physics, CAS. (Text and image by WANG Xueying)